Nicolas Desprat is graduated in fundamental physics from the University Pierre et Marie Curie. He holds a PhD in condensed matter and is currently a lecturer at the University of Paris. His research aims at understanding how physical constraints shape microbial communities.
Contact
L277
0144323468 L296
Laboratoire de physique
de l’Ecole normale supérieure
24 rue Lhomond 75005 PARIS
Research Interests
- Bacterial communities
- Host-pathogen interactions
- Collective behaviors in Chlamydomonas reinhardtii
- Saccharomyces cerevisiae in fluctuating environments
- Evolution in a spatially structured environment
Bacterial communities
Microcolony morphogenesis
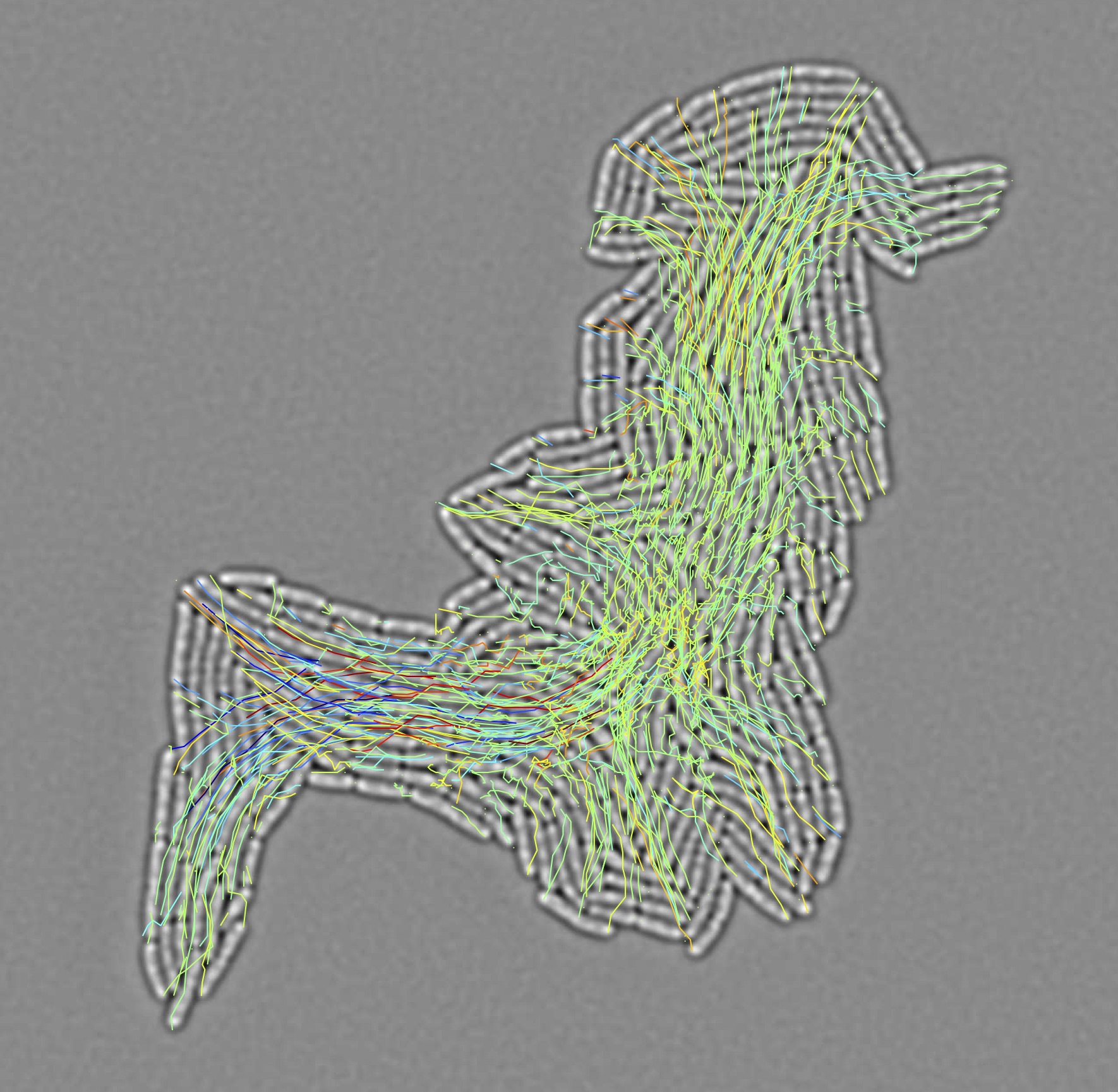
Bacterial populations interact with surfaces to establish dense, sessile communities. Surfaces are colonized by bacteria that first establish a 2D microcolony as a monolayer of bacteria, which then grows in the third dimension. Through single cell ablation experiments, force microscopy and mechanical modeling, we have shown that asymmetric adhesion favors the formation of 2D circular monolayers that maximize cell-cell interactions. Today, our work aims at understanding the cellular mechanisms responsible for polar adhesion. This work is done in collaboration with Christophe Beloin (Génétique des biofilms, Institut Pasteur) et Arnaud Gautier (Sorbonne Université).
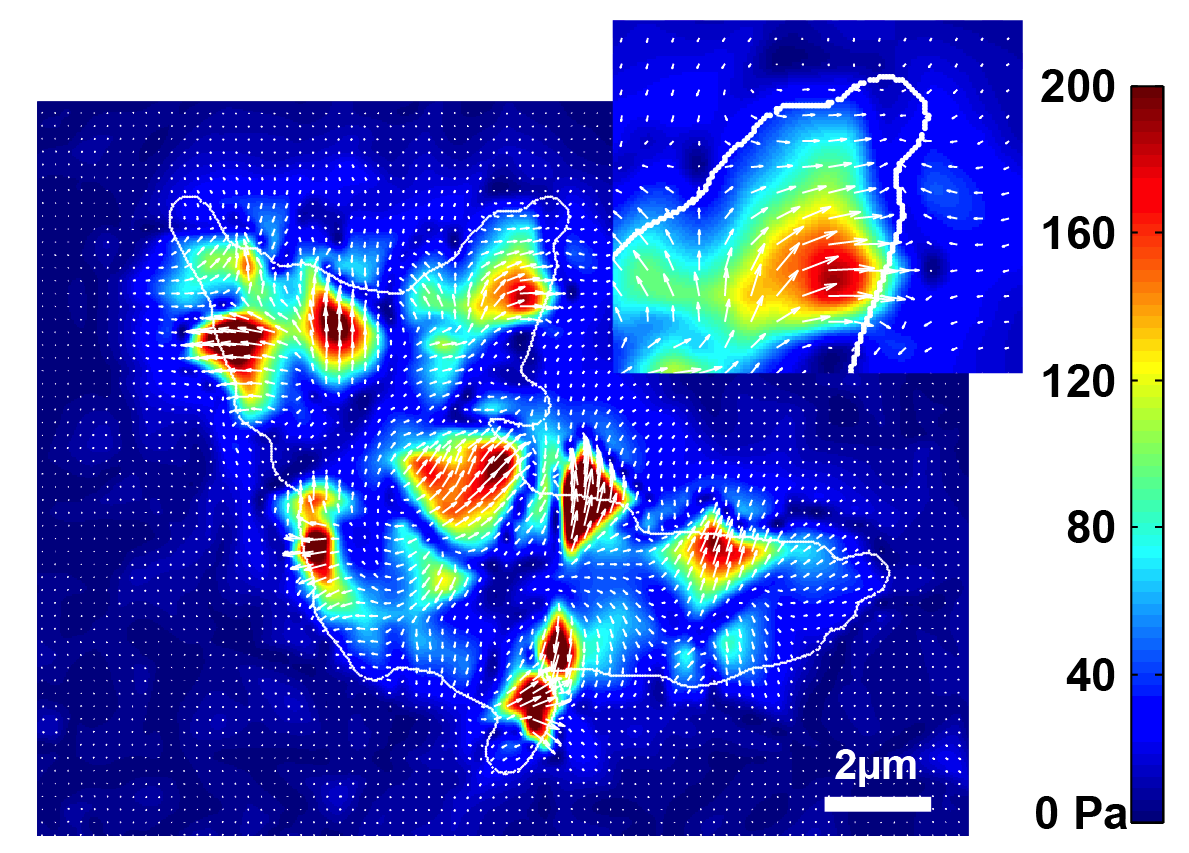
Public goods secretion
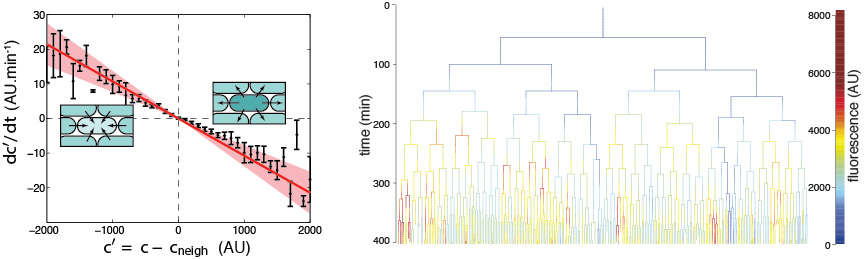
Many enzymes essential for cellular activity incorporate iron into their structure. The scarcity of soluble iron in aerobic environments has led most organisms to develop secretory and import mechanisms to deliver iron to the cytoplasm through siderophores (iron chelators). In a context where diffusion makes the investment of the metabolic cost of production vulnerable to exploitation by non-producing cells, we can ask ourselves what mechanisms can protect against such exploitation?
The bacterium Pseudomonas aeruginosa secretes a fluorescent siderophore, pyoverdine. We have shown that the concentration of pyoverdine in the periplasm of cells fluctuates over time and is widely distributed between cells. Nevertheless, local exchange mechanisms limit cell variability by adjusting the concentration balance between neighboring cells. This work has been done in collaboration with Thierry Mora (LPENS) and Isabelle Schalk (ESBS, UNISTRA).
Host-pathogen interactions
Vacuolar lifestyle of Listeria in epithelial cells
Listeria monocytogenes is an opportunistic pathogenic bacterium responsible for listeriosis. Its infectious mechanism is characterized by an intracellular life cycle. It enters the cells it infects by a mechanism close to endocytosis and finds itself enclosed in an internalization vacuole from which it must escape in order to replicate in the cytoplasm of the host cell. In collaboration with Alice Lebreton (IBENS) and Arnaud Gautier (Sorbonne Université), we were able to show that contrary to the commonly accepted view, Listeria was able to proliferate in the internalization vacuole before finding itself in contact with the cytoplasm of the host cell (Peron et al., PLoS Pathogens 2020). These results lead us to ask what is the role of this new intra-cellular niche in the infectious mechanism (collaboration with Marc Lecuit, Institut Pasteur).
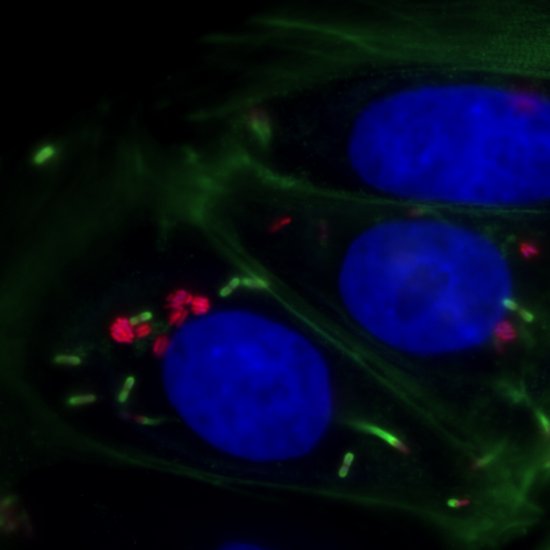
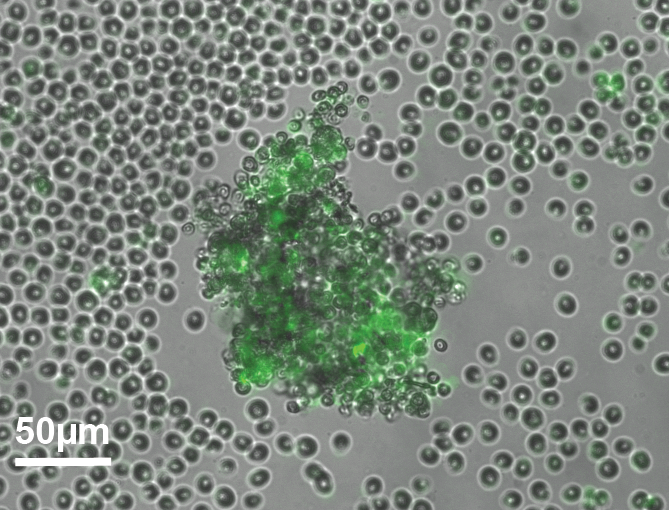
Collective behaviors in Chlamydomonas reinhardtii
Aggregation
The emergence of multicellularity is one of the major transitions in evolution. It has occurred several times in the history of life. Multicellularity has advantages, but intraspecific competition for environmental resources intensifies as group size increases. Therefore, the biological and physical conditions that initially led individual cells to form groups remain unclear. Chlamydomonas reinhardtii is a suitable model system for this study because it has multicellular cousins. This project proposes to explore the microscopic mechanisms underlying group formation and dispersal, which are prerequisites for understanding the ecology and evolution of multicellular life cycles. This project is carried out in collaboration with Antoine Danon (Sorbonne Université) and Raphaël Jeanneret (LPENS).
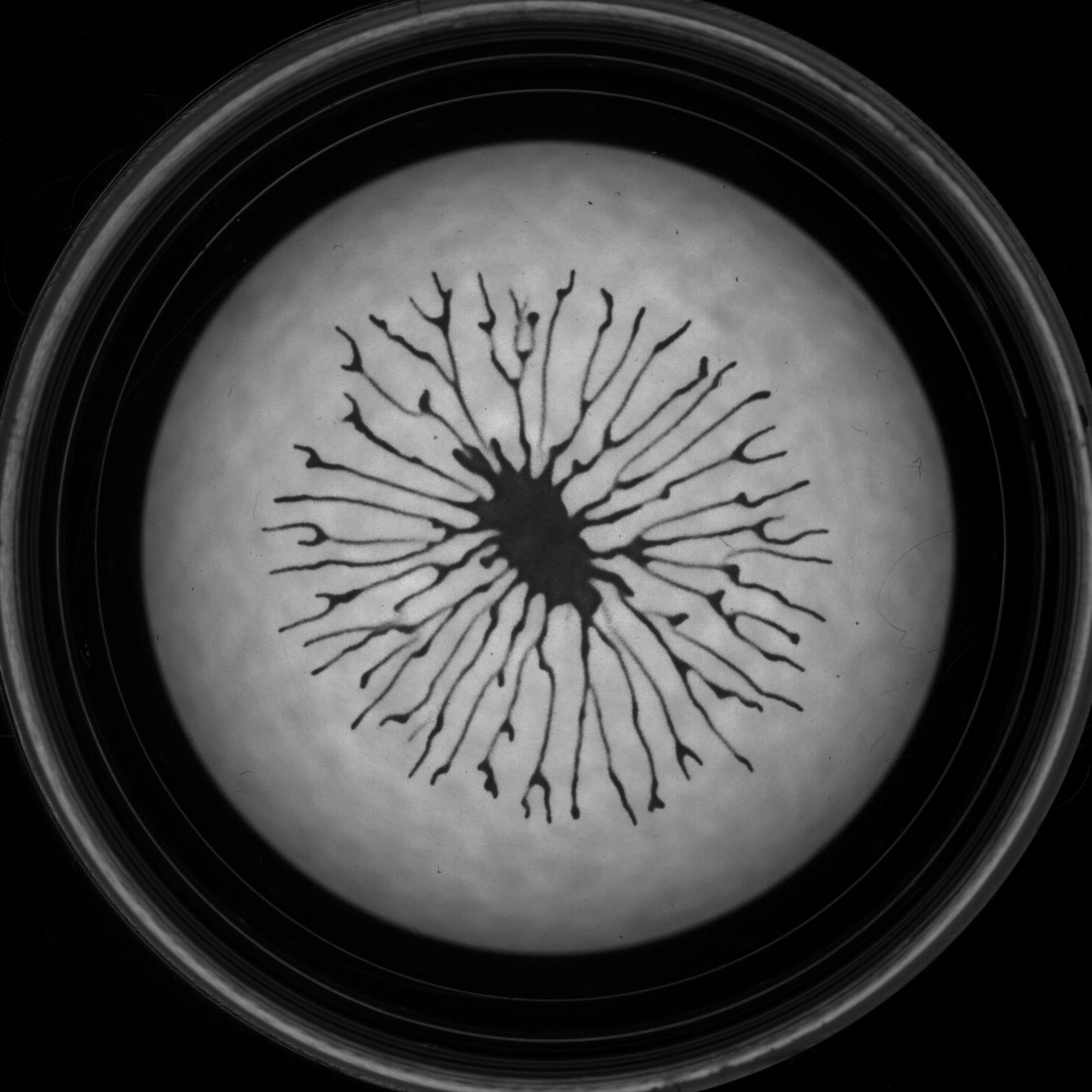
Phototaxis
The green alga Chlamydomonas reinhardtii is able to navigate in a light gradient to optimize the conditions for photosynthesis. With Raphaël Jeanneret (LPENS), we have recently reported a new collective behavior that emerges from the coupling between the algae density field and light propagation. This coupling leads to the formation of branched patterns.
Adaptation to fluctuating environments
Sacharomyce cerevisiae
In collaboration with Jean-Baptiste Boulé (MNHN), we have developed a fully automated bioreactor that allows us to impose either predictable or random environmental changes. We use it to measure physiological adaptation of the yeast Saccharomyces cerivisiae. The project aims at determining if there are mechanisms that can potentiate adaptation. Our bioreactor allows us to train a growing population for several months.
Evolution in a spatially structured environment
Echerichia coli in a temperature gradient
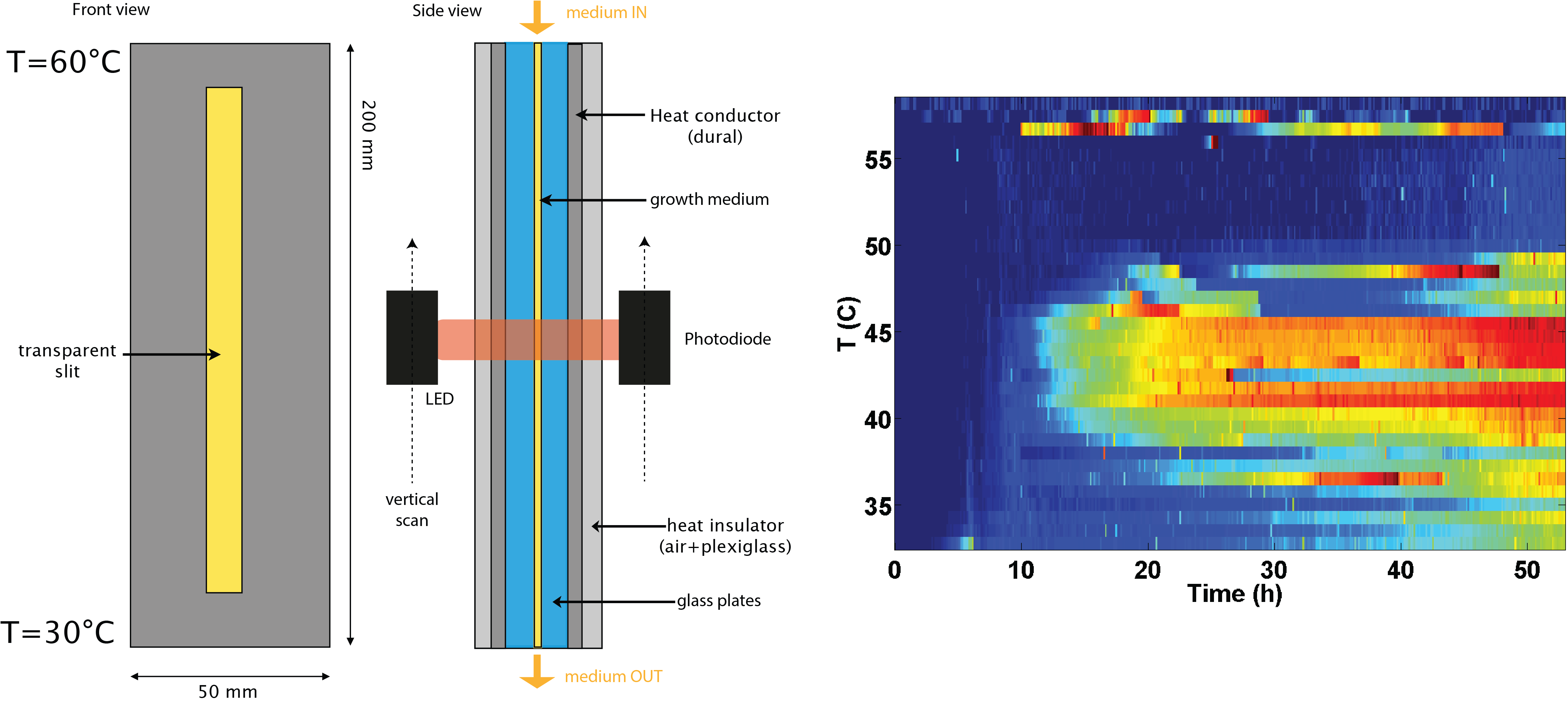
Experimental evolution experiments often involve sudden changes in conditions in a homogeneous environment. However, the spatial structure of the environment can facilitate adaptation. We have developed a chemostat to continuously grow a bacterial culture in a temperature gradient. The food is brought to the top of the column, which is maintained at a temperature of 60°C and the bacteria are flushed at the bottom, which is maintained at a temperature of 30°C. For E. coli, the maximum temperature of growth is around 45°C. Thus, the upper regions of our device offer favorable conditions with respect to nutrient concentration but are not permissive to growth.
Publications
Les 5 dernières publications :
- Peron-cane C., Leblanc J., Gautier. A., Desprat* N., Lebreton* A. [2020] Fluorescent secreted bacterial effectors reveal an intravacuolar replication compartment for Listeria monocytogenes. PLoS Pathogens 16(10):e1009001.
- Chekli, C. Peron-Cane, D. Dell’Arciprete, J-F. Allemand, C. Li, J-M. Ghigo, A. Gautier, A. Lebreton, N. Desprat* and C. Beloin* [2020] Visualizing the dynamics of exported bacterial proteins with the chemogenetic fluorescent reporter FAST Sci Rep. Sep 10:15791.
- Duvernoy MC, Mora T, Ardré M, Croquette V, Bensimon D, Quilliet C, Ghigo JM, Balland M, Beloin C, Lecuyer S, Desprat N. [2018] Asymmetric adhesion of rod-shaped bacteria controls microcolony morphogenesis. Nat Commun. 2018 Mar 16;9(1):1120
- Hormoz S, Desprat N, Shraiman B. [2015] Inferring Epigenetic Dynamics from Kin Correlations. PNAS doi:10.1073/pnas.1504407112
- Gallie J, Libby E, Bertels F, Remigi P, Jendresen CB, Ferguson GC, Desprat N, Buffing MF, Sauer U, Beaumont UJE, Martinussen J, Kilstrup M, Rainey PB [2015] Bistability in a Metabolic Network Underpins the De Novo Evolution of Colony Switching in Pseudomonas fluorescens. PLoS biology 13:e1002109.
Curriculum Vitae
Situation actuelle
- Associate Professor – The University of Paris
- Team ABCD: Physics of Microbes (LPENS)
Cursus
- 2005-2007 : INSERM Postdoctoral fellow at Curie Institute (UMR168) in Emmanuel Farge’s lab (Mechanics and genetics of embryonic development). Interplay between developmental gene expression and morphogenetic movements in gastrulating drosophila embryos.
- 2001-2005 : PhD University of Paris under the supervision of Atef Asnacios (MSC) : Single cell rheology
- 2000 : Master degree in solid state physics and condensed matter (Sorbonne University)
Télécharger le CV complet : ![]()
Teaching
- Stanford Overseas Program in Paris: Optics and Electromagnetism
- Associate Professor in Physics (The University of Paris)